Quantum Physics
Understanding electron scattering with ultra-long-range Rydberg molecules
22 October 2019
Photo: AG Schmelcher
Ultra-long-range Rydberg molecules consist of a highly excited Rydberg atom with a loosely bound electron and at least one atom in its ground state located within the orbit of the Rydberg electron. Due to the molecule's size and the large extent of the electronic orbit, they form with huge internuclear distances up to a thousand times larger than in usual molecules. Accordingly, they posses giant dipole moments and are specifically useful to study fundamental properties of atomic interactions.
Recently, we have collaborated with scientists at the University of Stuttgart and succeeded in measuring spectra of ultra-long-range Rydberg molecules made of ultra-cold Rubidium with such a high precision that it was possible to infer the structure of negative ions. Tuned carefully, the system of Rydberg electron and neutral ground-state atom temporarily forms a negative ion. The advantage is that the ions remains ultra-cold, which is very hard to achieve by other means. This experiment has established a promising route to study cold ions in the future.
We were also involved in an experiment in Oklahoma, where it was possible to verify triatomic ultra-long-range Rydberg molecules in spectra of ultra-cold Cesium. In this type of molecule, an additional ground-state atom is bound by the Rydberg electron. They come in different molecular geometries, such as linear, where the Rydberg atom is located in the middle between the two ground-state atoms, or orthogonal, resembling a rectangular triangle. The geometry depends on the molecular energy. Interestingly, the two ground-state atoms have at no time direct contact to each other. The Rydberg electron, however, mediates an exchange between the two, which leads to effective three-body interactions in the molecular system. This effect was now verified for the very first time. This is an exciting example for the great opportunities that ultra-long-range Rydberg molecules offer to study fundamental interactions in atomic systems.
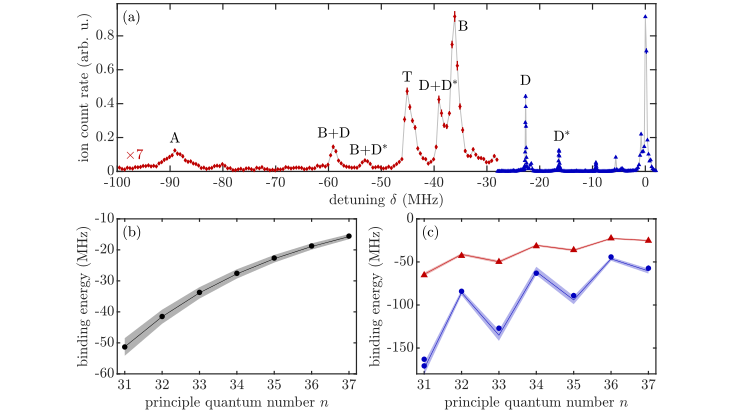
The spectrum of an ultra-cold atomic cloud of Rubidium (top) shows typical peak, which can be assigned to ultra-long-range Rydberg molecules (named by the various capital letters). The energetic position of the peaks over various quantum numbers allows to infer underlying interactions, here for s-wave scattering (bottom left) and p-wave scattering (bottom right).


